A mandatory part of the cosmetics packaging without which selling any product would be impossible is proper labeling. Cosmetic labeling is made according to a set of rules and regulations devised by the country in which the products are sold.
Regardless of the country of origin, it is essential that cosmetic brands follow the guidelines of their consumer continent when it comes to launching their products in one of its countries.
Although America follows the FDA guidelines for cosmetics labeling, the rules and regulations for the European Union and the United Kingdom are different. So, if you are planning to launch your products in these continents, it is important to understand the process of labeling in the EU and UK.
Therefore, we will walk you through the details of introducing your product in the UK and Europe in this article.

Are UK Cosmetics Labeling Guidelines Different from EU?
Yes, the UK cosmetics labeling guidelines are slightly different from that of EU guidelines. The guidelines of the UK show some leniency in contrast to the strict policies of the EU.
While points like brand name, unit of measurement, general warnings, how-to instruction, and expiration date are the same in both, below are the key differences.
Information About the Responsible Person
The information like name, address, and contact of the responsible person (we will discuss it later) is printed on the label of the cosmetic jar in the EU. However, this information is mentioned on the secondary packaging of the cosmetic jars in the UK.
Information About the Product
Just like RP’s information, information like name, benefits, and purpose of the product is to be mentioned on the label in Europe and it can be skipped on UK cosmetics labels.
Compatible Language
European Union has 55 countries in which 200 languages are spoken across the continent. So, the compatible language of the label depends upon the country it is sold. On the other hand, all labels in the UK are either in English, Welsh, and Irish.
Notification Portal
Before selling the cosmetics in the market, the information on the products is notified through notification portals of the respective continent.
What is the Responsible Person (RP)?
As the name implies, the responsible person is the person, firm, or business responsible for ensuring the safety and law compliance of cosmetic products.
In general, the manufacturer, importer, transporter, or brand owner can be the Responsible Person. The RP for British products must be a person from the UK and for European products must be from Europe.
The duty of the responsible person is to check information about the product, information about the RP, warning signs, instructions of use, quantity, expiration date, ingredient list, claims of the product, and precautions on the label of the cosmetics products in addition to ensuring that no harmful material is used in the manufacturing process and the product is notified in the notification portal as well.
Furthermore, the responsible person has to update the information about the product and formatting of the labels according to the recent guidelines. You can check more details about the Responsible Person on Gov.UK portal.
What is a Product Information File (PIF)?
Product Information File is a document in which all the information about the specific product is written in detail. The purpose of this file is to allow quick and thorough inspection of the product anytime. Moreover, it is important to note that each product of a brand has its separate PIF.
The Responsible Persons keep this PIF with them and present it to the authorities when needed. PIF is held for 10 years after the release of the product and it comprises the following information,
- Reports about safety, experiments, and results of the products
- General information including the name and benefits of the product
- Details about compliance of the product with GMP
- The reality of the claims of the product
Cosmetic Product Safety Regulation
Whether it is cosmetics launched in the UK or EU, here are some important product safety regulations for both.
1. Safety Assessment
Safety assessment is the process in which the product is tested on humans or animals to determine its safety of utilization on human skin. It helps in knowing the potential reactions, effects, and side effects of the product.
Also, note that it is preferred not to test the product on animals.
2. Ingredient Safety
First of all, it is important to use ingredients regarded as safe in their recommended concentrations in the formulation of the product.
Both micro and macro nutrients must be harmless for humans to be used in cosmetic products and their safety and purity should be tested after the formulation of the product as well.
3. Microbiological Safety
During the formulation and transport of the products, microbes including bacteria, viruses, and fungi can find their way into the cosmetic jars.
Thus, the product must be formulated and stored in a sterilized environment where there is little to no risk of microbial contamination. Additionally, a microbiological safety test is conducted before the release of the product in the market.
Good Manufacturing Practice (GMP) Compliance
Good manufacturing practice is the standard process including different steps that are involved in the production of the product. Flowing standard ISO 22716. These practices involve,
- Hiring employees who are well aware of their responsibilities and skilled enough to fulfill them
- Maintaining the hygiene of the production and packaging unit of the cosmetics
- Regular service and cleaning of the equipment used for product formulation, mixing, and packaging
- Use of sterilized ingredients
- Adequate product safety and quality tests throughout product manufacturing
- Each batch of the products has specific labels and batch numbers
- Ensuring proper disposal of the wastes
- Updating the product information throughout the process
- Noting the changes and reason for the changes in product formulation
- Storing and distributing the products safely
Cosmetic Product Labeling
We have said it before and will say it again, FDA-complaint cosmetic product labeling is as important as the product formulation itself if you want your product to sell well in the market. Each cosmetic label must have information about,
- Brand and product name
- Product Type
- Best before duration
- How-to use instruction
- Ingredient list
- Country of origin
- Quantity of the product
- Batch number
- Details of a responsible person
- Precautions and warning signs of the products
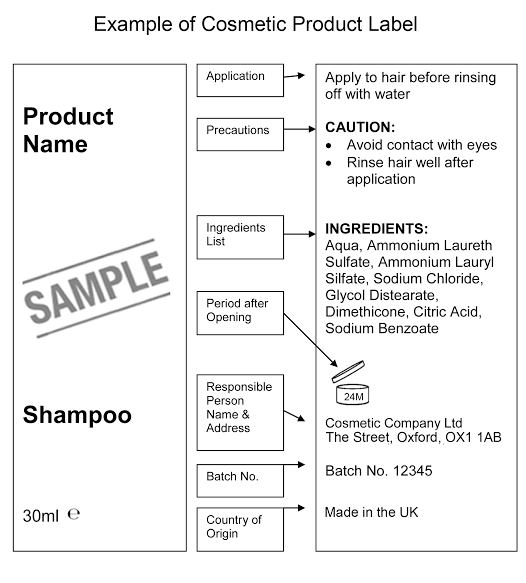
Additionally, you can follow cosmetic packaging tips and use labels to flaunt your beauty packaging designs.
Cosmetic Brand Advertising/Claims
Many brands claim the benefits of the product which are not offered by the product in reality. Some of the examples include calling the product “organic” when its ingredients are not up to the definition of organic.
Similarly, some products claim that they have certain effects while their ingredient list says otherwise. So, false advertisements and claims can cause cosmetic brands to face legal action.
Cosmetic Product Notification Portal (CPNP)
Before launching any product in the cosmetics market for sale, it is mandatory for the Responsible Person of the brand to notify the details of each product to the Cosmetic Product Notification Portal. The details include,
- Ingredients and their safety
- Testing reports of the product
- Benefits and uses of the product
Record Keeping for Compliance
Documentation is one of the most necessary steps in obeying the UK or EU regulations regarding cosmetic product manufacturing and release.
Record Keeping for Compliance means ensuring that you have the details of every step from production to using the right material for cosmetic packaging and delivery of the cosmetic products.
This record-keeping comes into use when your brand is audited by authorities to know if it is compliant with the laws.
Public Accessibility of Product Information
While the ingredient list, brand information, and responsible person’s credentials are already mentioned on the cosmetics label, it is essential to make the following product information accessible to the public if anyone wants it.
- Details of side effects or unwanted effects of the product
- Content of ingredients in a certain unit of the product
- Qualitative information about the product
Risks Associated with Non-Compliance with Cosmetics Guidelines
In case you fail to follow the cosmetics guidelines mentioned in this article, you can face the consequences given below.
1. Penalties and Fines
The beauty brands that are found going against the rules and regulations of cosmetic labeling as set by the UK and EU are liable to pay penalties and fines to the authorities. The fine in the UK can be a few hundred or several thousand pounds while it is up to a million euro in the EU.
2. Cosmetic Product Recall
The cosmetic labeling must also be compliant with the guidelines of the continent in question. In the other cases, products are recalled even after being launched in the market and the brand cannot sell the products until its labeling is compliant with the regulations.
3. Legal Liability
If the product has fall claims or unsafe ingredients that result in any kind of loss for the consumer, the cosmetic company can be sued. Consequently, legal liability comes in the form of fines and compensation to the affected person.
4. Limited Market Access
The products compliant with UK regulations can be sold in England, Ireland, and a few more countries only if they follow British guidelines. A similar is the case with products released in the European market.
However, if the guidelines are not followed accurately on an international level, there are high chance that you won’t be able to sell your products in an extensive market.
Conclusion
The formulation of the product and safety testing form the basics of regulatory compliance for releasing any new item in the industry. However, UK or EU-compliant cosmetic product labeling serves as the step without which packaging for cosmetics is incomplete.
Without a proper label, no beauty brand can sell or even launch its products in the market. Furthermore, following the regulatory guidelines enthusiastically can be difficult. Therefore, we are here to present you with everything you need for cosmetic branding.

